U.S. Aortic Stenosis Disease Prevalence & Treatment Statistics
- Heart disease is the leading cause of death in the U.S., killing more than 600,000 Americans each year.1
- More than five million Americans are diagnosed with heart valve disease each year.2
- Heart valve disease can occur in any single valve or a combination of the four valves, but diseases of the aortic and mitral valves are the most common.
- Calcific aortic stenosis is the most common cause of aortic stenosis (AS).3
- While up to 1.5 million people in the U.S. suffer from AS, approximately 500,000 within this group of patients suffer from severe AS. An estimated 250,000 patients with severe AS are symptomatic.4-6
- An echocardiogram (echo) is the primary imaging test used to diagnose severe AS.
- Without an aortic valve replacement (AVR), as many as 50 percent of patients with severe AS will not survive more than an average of two years after the onset of symptoms.7
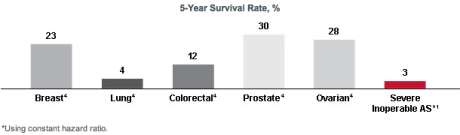
- The predicted survival of inoperable patients with severe AS who are treated with standard non-surgical therapy is lower than with certain metastatic cancers.8
- Studies show that severe AS is undertreated. At many hospitals, more than 50 percent of patients that receive an echo and show the presence of the disease are not referred to a surgeon to be evaluated for an AVR. The absence of chest pain symptoms and overestimating risks associated with the AVR procedure have been identified as some of the reasons lack of patient referrals occur.9-11
- An estimated 80,000-85,000 AVR procedures are performed every year in the U.S.
Transcatheter Aortic Valve Replacement (TAVR)
- The first successful TAVR procedure was performed in France on April 16, 2002, by Alain Cribier, M.D., University Hospital Charles Nicolle in Rouen, France.
- The Edwards SAPIEN transcatheter valve received CE Mark for commercial sale in Europe in 2007.
- To date, more than 45,000 patients have been implanted with Edwards’ transcatheter valves by multi-disciplinary heart teams worldwide.
- The Edwards SAPIEN Transcatheter Heart Valve is FDA-approved for the treatment of both inoperable and high-risk patients with severe, symptomatic calcified native aortic valve stenosis in need of an aortic valve replacement.
- The Edwards SAPIEN Transcatheter Heart Valve delivered via an incision in the leg, or the transfemoral procedure, was first approved in November 2011 by the FDA as a therapy for inoperable patients.
- In October 2012, the FDA approved an expanded indication to enable the treatment of high-risk patients. The FDA also approved a new delivery method called the transapical procedure for high-risk patients without suitable access through their leg artery. During this procedure, the valve is inserted via an incision between the ribs and through the bottom end of the heart called the apex.
- The Edwards SAPIEN valve is designed to replace a patient’s diseased native aortic valve without the need for open-chest surgery and without stopping the patient’s heart.
- Data from both the high risk and inoperable study groups in The PARTNER Trial were published in four separate manuscripts in The New England Journal of Medicine.12-15
- Certain inoperable patients with severe, symptomatic native valve aortic stenosis are not candidates for TAVR due to the presence of other co-existing medical conditions or disease processes that would that would make them too sick to experience the expected benefit from fixing their aortic stenosis.
- The Edwards SAPIEN Valve is the only FDA-approved TAVR therapy in the U.S.16
- The PARTNER Trial was the world’s first randomized controlled trial of TAVR, and importantly, cardiac surgeons and interventional cardiologists were brought together in a clinical trial to collaborate to evaluate and treat patients.
References
- Miniño AM, Murphy SL, Xu J, Kochanek KD. Deaths: Final data for 2008. National Vital Statistics Reports; vol 59 no 10. Hyattsville, MD: National Center for Health Statistics. 2011.
- Nkomo V, Gardin M, Sktelton T, et al. Burden of valvular heart diseases: a population-based study (part 2). Lancet: 2006:1005-11.
- Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation. 2005;111:920–925.
- Bach D, Radeva J, Birnbaum H, et al. Prevalence, Referral Patterns, Testing, and Surgery in Aortic Valve Disease: Leaving Women and Elderly Patients Behind. J Heart Valve Disease. 2007:362-9.
- Iivanainen A, Lindroos M, Tilvis R, et al. Natural History of Aortic Valve Stenosis of Varying Severity in the Elderly. Am J Cardiol. 1996:97-101.
- Aronow W, Ahn C, Kronzon I. Comparison of Echocardiographic Abnormalities in African-American, Hispanic, and White Men and Women Aged >60 Years. Am J Cardiol. 2001:1131-3.
- Otto, CM. Timing of Aortic Valve Surgery. Heart 2000;84(2):211-8.
- Leon M, Smith C, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597-1607.
- Bach DS, Siao D, Girard SE, et al. Evaluation of patients with severe symptomatic aortic stenosis who do not undergo aortic valve replacement: The potential role of subjectively overestimated operative risk. Circ Cardiovasc Qual Outcomes 2009;2:533-539.
- Bach DS. Prevalence and Characteristics of Unoperated Patients with Severe Aortic Stenosis. J Heart Valve Dis 2011;20:284-291.
- Xu JQ, Kochanek KD, Murphy SL, et al. Deaths: final data for 2007. [PDF - 3.41MB] National Vital Statistics Reports 2010;58(19).
- Leon MB, Smith CR, Mack M, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187-98.
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597-1607.
- Makkar RR, Fontana GP, Jilaihawi H, et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med. 2012;366(18):1696-704.
- Kodali SK, Williams MR, Smith CR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;366(18):1686-95.
- U.S. Food and Drug Administration (November 2, 2011). FDA approves first artificial aortic heart valve placed without open-heart surgery [press release]. Retrieved from http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm278348.htm.
- U.S. Food and Drug Administration (October 19, 2012). FDA expands approved use of Sapien artificial heart valve [press release]. Retrieved from http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm323478.htm?source=govdelivery